Abstract
Background: Treatment for chemotherapy-induced thrombocytopenia (CIT) has generally been limited to modification of chemotherapy regimen (dose reduction, treatment delay, omission, and/or discontinuation of one or more agents) or platelet transfusions, which provide only transient benefit and can be associated with adverse events. With data from a phase 2 prospective study and retrospective studies of the thrombopoietin receptor agonist romiplostim, National Comprehensive Cancer Network® (NCCN®) guidelines now include consideration of romiplostim for treatment of CIT. Two pivotal phase 3 randomized controlled trials of romiplostim to treat CIT in patients with solid tumors are underway.
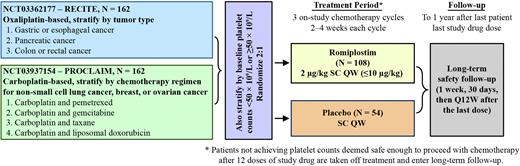
Trial design: Patients (≥18 years old) with platelet count ≤85×109/L and CIT from a prior regimen who are slated to receive (trial 1) oxaliplatin-based chemotherapy for esophageal, gastric, pancreatic, or colorectal cancer (NCT03362177) or (trial 2) carboplatin-based chemotherapy for non-small cell lung, ovarian, or breast cancer (NCT03937154) will be stratified by baseline platelet count and tumor type (trial 1) or specific carboplatin regimen (trial 2). Patients will be randomized 2:1 to receive romiplostim or placebo, respectively. Weekly study drug will be initiated at 2 μg/kg subcutaneously and titrated by 1 μg/kg (maximum of ≤10 μg/kg) to a target platelet count of ≥100×109/L. When a platelet count of ≥100×109/L is achieved or at Week 4, if deemed appropriate by the investigator, chemotherapy will be initiated. Treatment with study drug will be stopped after 12 weeks if platelet count ≥100×109/L (or a platelet count deemed safe to proceed with chemotherapy) is not achieved. For each trial, once 81 of the 162 anticipated patients have completed 3 chemotherapy cycles, an interim analysis will be conducted.
Endpoints: For both studies, there is the same primary endpoint, i.e., thrombocytopenia-induced dose modification (ie, dose reduction, delay, omission, and/or discontinuation) of any myelosuppressive agent in the second and third cycles of the planned chemotherapy regimen, as adjudicated by an independent committee (oncologists and a biostatistician). Secondary endpoints include safety, survival, platelet response (proportion achieving response, time to response), platelet nadir (depth), grade ≥2 bleeding rate (adjusted for duration), and incidence of platelet transfusion. Previously presented at ESMO 2022, FPN 1628TiP, Al-Samkari et al. Reused with permission.
Disclosures
Al-Samkari:argenx: Consultancy; Novartis: Consultancy; Sobi: Consultancy, Research Funding; Amgen: Research Funding; Forma: Consultancy; Rigel: Consultancy; Moderna: Consultancy; Agios: Consultancy, Research Funding; Dova: Consultancy, Research Funding. Geredeli:Bayer: Honoraria; Amgen: Honoraria; Roche: Honoraria; BMS: Honoraria; Pfizer: Honoraria; Takeda: Honoraria; Astellas: Honoraria; AstraZeneca: Honoraria; Merck: Honoraria; Eczacibasi: Honoraria; Novartis: Honoraria; Deva: Honoraria. Arslan:Roche: Honoraria, Other: Grants and contracts; Novartis: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; BMS: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; Merck Sharpe & Dohme: Honoraria, Other: Grants and contracts; AstraZeneca: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; Nektar: Other: Grants and contracts; Johnson & Johnson: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; Lilly: Honoraria, Other: Grants and contracts; Astellas: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; Amgen: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; Teva: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; Bayer: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; Henlius: Other: Grants and contracts; Yuhan: Other: Grants and contracts; Daiichi Sankyo: Other: Grants and contracts; Pfizer: Honoraria, Other: Grants and contracts, Support for attending meetings and/or travel, Participation on a Data Safety Monitoring Board or Advisory Board; Sanofi-Aventis: Other: Grants and contracts; Incyte: Other: Grants and contracts. Fernandez:Amgen: Consultancy, Speakers Bureau; Celgene: Consultancy; Servier: Consultancy; BMS: Speakers Bureau; Merck Serono: Speakers Bureau; Rovi: Speakers Bureau; Roche: Other: Travel, Accommodations, Expenses; Sanofi: Other: Travel, Accommodations, Expenses; Merck: Other: Travel, Accommodations, Expenses. Ciuleanu:A&D Pharma: Consultancy, Other: Travel, Accommodations, Expenses; Amgen: Consultancy, Other: Travel, Accommodations, Expenses; Astellas Pharma: Consultancy, Other: Travel, Accommodations, Expenses; AstraZeneca: Consultancy, Other: Travel, Accommodations, Expenses; Boehringer Ingelheim: Consultancy, Other: Travel, Accommodations, Expenses; BMS: Consultancy, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Other: Travel, Accommodations, Expenses; Lilly: Consultancy, Other: Travel, Accommodations, Expenses; Merck Serono: Consultancy; Merck Sharp & Dohme: Consultancy, Other: Travel, Accommodations, Expenses; Novartis: Consultancy, Other: Travel, Accommodations, Expenses; Pfizer: Consultancy, Other: Travel, Accommodations, Expenses; Roche: Consultancy; Sanofi: Consultancy, Other: Travel, Accommodations, Expenses; Servier: Consultancy, Other: Travel, Accommodations, Expenses; Ipsen: Other: Travel, Accommodations, Expenses; Merck: Other: Travel, Accommodations, Expenses; Roche/Genentech: Other: Travel, Accommodations, Expenses. Bowers:Amgen: Current Employment, Current equity holder in private company. Armas:Amgen: Current Employment, Current equity holder in private company. Scotte:Amgen: Honoraria, Research Funding; Mylan: Honoraria; Roche: Honoraria, Research Funding; Mundipharma: Honoraria; Leo Pharma: Honoraria; Tesaro: Honoraria; Helsinn: Honoraria; Tilray: Honoraria; Vifor Pharma: Honoraria; Pfizer: Honoraria; BMS: Honoraria. Bunn:AstraZeneca: Honoraria; Merck: Honoraria; Genentech: Honoraria; Lilly: Honoraria; BMS: Honoraria; Takeda: Honoraria; CStone: Honoraria; Ascentage: Honoraria; Imidex: Honoraria; Verastem: Membership on an entity's Board of Directors or advisory committees. Kuter:BioCryst: Consultancy, Research Funding; UCB: Consultancy, Research Funding; Caremark: Consultancy; CRICO: Consultancy; Daiichi Sankyo: Consultancy; Dova: Consultancy; Genzyme: Consultancy; Incyte: Consultancy; Kyowa Kirin: Consultancy; Merck Sharp & Dohme: Consultancy; Momenta: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Platelet Disorder Support Association: Consultancy; Sanofi: Consultancy; Shionogi: Consultancy; Shire: Consultancy; Up-To-Date: Consultancy; Zafgen: Consultancy; Rubius: Current holder of stock options in a privately-held company; Platelet Biogenesis: Consultancy; Cellularity: Consultancy; Cellphire: Consultancy; Hengrui: Consultancy; Takeda (Bioverativ): Consultancy, Research Funding; Rigel: Consultancy, Research Funding; Protalex: Consultancy, Research Funding; Immunovant: Consultancy, Research Funding; BMS: Consultancy, Research Funding; argenx: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Alnylam: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Actelion (Syntimmune): Consultancy, Research Funding; Kezar: Research Funding; Principia: Consultancy, Research Funding. Soff:Amgen: Research Funding; Janssen: Research Funding; Scientific Affairs: Research Funding; Dova: Research Funding; Hengrui USA: Research Funding.
OffLabel Disclosure:
Romiplostim is under investigation for use in chemotherapy-induced thrombocytopenia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal